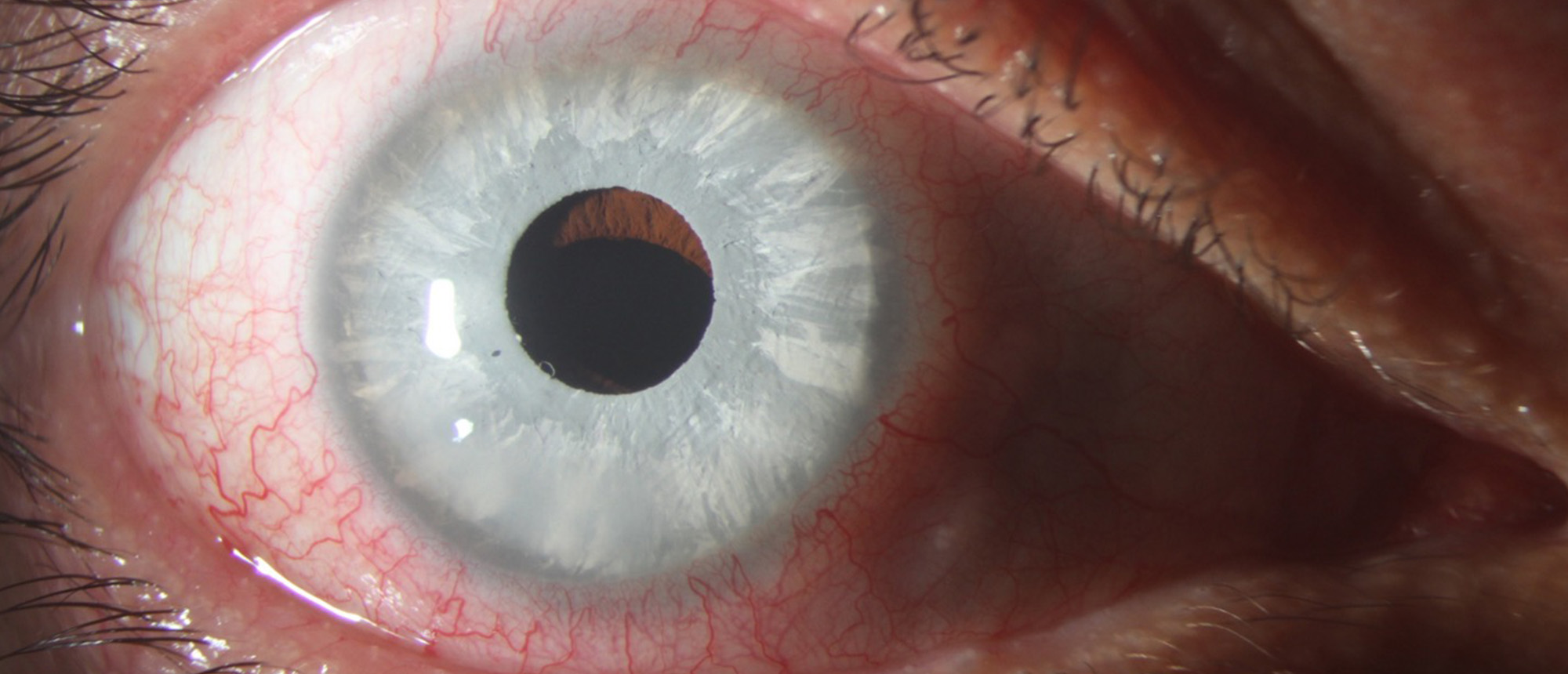
Cosmetic Iris Implant
A patient presented for bilateral blurred vision and eye pain of 3 days duration. The patient had previously undergone bilateral LASIK and cosmetic iris implantation. He was found to have decreased vision of 20/800 on the right eye and could see the 20/200 E when held at his face on the left eye. His intraocular pressures were elevated in both eyes, and he had significant corneal edema. He underwent bilateral explantation of the implants, and he required a cataract extraction and DSAEK surgery in the left eye for corneal edema. At last follow up, the vision was 20/800 in the left eye, with findings consistent with pressure-induced stromal keratitis. Cosmetic iris implants are not available for use in the United States or European Union, however they are available in other countries. There have been many cases of ocular complications from these implants, including glaucoma, corneal edema, uveitis, and cataract. Most patients ultimately require explantation, which can be done in many ways described in the literature. Because the complication spans across multiple sub-specialties, ophthalmologists in any field must familiarize themselves with the presenting symptoms of complications and indications for removal.
Presentation Date: 03/16/2023
Issue Date: 03/31/2023
Please log in or click on ENROLL ME to access this course.